Hello all,
This came from another forum. I am attempting to calculate and generate entropy data based on EPW and create an hourly plot. I am wondering if this can be used in atmospheric science, meteorology, or weather forecasting. (theoretical base).
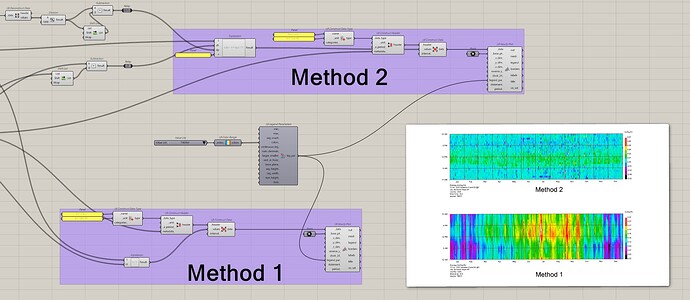
Below are the two calculation/hypothesis methods:
**Method 1:
Relationship between Enthalpy and Entropy:
TΔs = Δh − vΔp
where:
- T is the temperature (k)
- Δs is the change in entropy
- Δh is the change in enthalpy
- v is the specific volume
- Δp is the change in pressure
In this method, the specific volume v and the pressure change Δp is ignored, which means the direct relationship between Enthalpy (H) and Temperature (T) is only considered. This can be simplified to:
Δs = Δh/T
To calculate the change in entropy ΔS:
Assuming H is the change in enthalpy per unit mass (kJ/kg), and the initial state has a change in enthalpy Δh=16.63 kJ/kg, with the initial state’s entropy being zero (reference state), it can be written as:
ΔS = H/T
**Method 2:
In this method the specific volume v is ignored (assume = 1), Δh and Δp is done by substrate the value of previous hour.
Therefore:
TΔs = Δh − 1 * Δp
Please let me know what you thinks, and if it even make sense!
Entropy.gh (52.2 KB)